ABSTRACT
The computers are everywhere. They are a wonderful tool and without a shadow of doubt make things possible which were only a dream not too long ago. Thus the increased use of computers into the engineering curricula is a welcome and timely change. The use of computers in education usually includes the software that is supplied with the textbooks. But for most part this amounts to a piece-meal approach to computer assisted instruction at best. The 'pieces' include the software that comes with the textbook, equation solver(s) in the computer lab, special optimization routines, spreadsheets, plotting software programs and finally the word processors. What is desirable is a tool which has all of these capabilities under one roof and which works with almost any word processor. At Southern Illinois University-Carbondale the author has implemented, multi-tasking computer assisted engineering instruction via such a program which is relatively new, user friendly and commercially available, in many of his undergraduate courses and design electives. Several illustrative uses of the program are presented in this paper.
While the computer aided education is desirable, if not essential, there are some concerns which the author addresses. Computer is only a tool and hence is not a substitute for critical thinking. While computers are great for exploration and for performing "what-if" types of analyses, they can hinder the process of creative query and common-sense compatibility. Armed with computers the students seem eager to get answers fast and to trust those results without question or hesitation. Occasionally when they are not certain about the results, they have a difficult time in rectifying any potential conceptual or mathematical flaws. Some examples and proposed remedies are discussed with a few concluding remarks about an article which the author read, "Beware of the cosmetic engineer!."
CELEBRATION OF COMPUTERS IN ENGINEERING EDUCATION
As outlined in the abstract above, in this paper two aspects of computers in engineering education are addressed. One is celebration of computers in engineering and the other is the caution one should exercise in using computers. For the first part regarding the computer usage, the author implemented comprehensive usage of a relatively new; user-friendly; commercially available computational tool for undergraduate in-class instruction. The application of this software frees up students from the traditional and mundane task of researching raw physical and chemical data necessary for performing engineering calculations thus enabling them to focus more closely on design and analytical aspects of curriculum. Author has effectively used this software both on the job in private industry and also for undergraduate instruction at the present university and at the University of Wisconsin-Milwaukee in the capacity as an adjunct associate professor.
The main objective of the this work was to develop comprehensive and innovative learning opportunities for in-class, computer assisted engineering solutions and simulations for a variety of situations including the open-ended design assignments. These in-class simulations made it possible to carry out interactive; "what-if" types of analyses which are particularly crucial in engineering practice. The software program that the author used is called, "Engineering Equation Solver" or EES (pronounced 'ease') [1].
While the program allows for simultaneous solutions of hundreds of non-linear equations in any algebraic form it does a lot more than that. What is unique about EES, as compared to other solvers, is that it comes equipped with built-in functions for all thermodynamic and physical properties for an exhaustive list of materials that are used in our industries. The examples of properties include enthalpy; entropy; specific heats; viscosity; thermal conductivity. The effect of pressure on the gas transport properties is estimated in addition to that of temperature. The materials include various ideal gases; psychrometric air; refrigerants (including new refrigerants that are in compliance with the clean air act and the old refrigerants that are being phased out); steam; ice. Additionally EES allows for addition of fluids to the property data base.
The motivation for this work arose out of author's experience in teaching a variety of courses in Mechanical engineering and based on principal research investigations in private industry. The use of computers in education is on the rise and it typically includes the software that is supplied with the textbooks. But for most part this amounts to a piece-meal approach to computer assisted instruction at best. The 'pieces' include the software that comes with the textbook; equation solver(s) in the computer lab; special optimization routines; spreadsheets; plotting software programs and finally the word processors. Thus it was desirable to have a tool like EES which has all of these capabilities under one roof and which works with almost any word processor including Microsoft Wordª. Additionally, once mastered this program can be used for a number of engineering courses including the design electives.
It is well known that in the engineering education community, especially for the undergraduate courses such as thermodynamics and heat transfer, in order to learn the materials it is necessary for the students to work problems. However, much of the time and effort required to solve problems results from looking up property information and solving the appropriate equations. Once the student is familiar with the use of property tables (still a very important aspect however), further use of the tables does not contribute to the student's grasp of the subject, nor does the use of algebra.
The time and effort required to do problems in the conventional manner may actually detract from learning of the subject matter by forcing the student to be concerned with the order in which the equations should be solved (which really does not matter in EES) and by making parametric studies too laborious. Interesting practical problems that may have implicit solutions, such as those involving both thermodynamic and heat transfer considerations, are often not assigned because of their mathematical complexity. EES allows the user to concentrate more on design by freeing her or him from mundane chores.
EES is particularly useful for design problems in which the effects of one or more parameters need to be determined. The program provides this capability with its Parametric Table, which is similar to a spreadsheet. The user identifies the variables which are independent by entering their values in the table cells. EES calculates the values of the dependent variables in the table. The relationship of the variables in the table can then be displayed in plots. With EES once set up, it is no more difficult to do design problems that it is to solve a problem for a fixed set of independent variables.
EES PROGRAM ILLUSTRATION
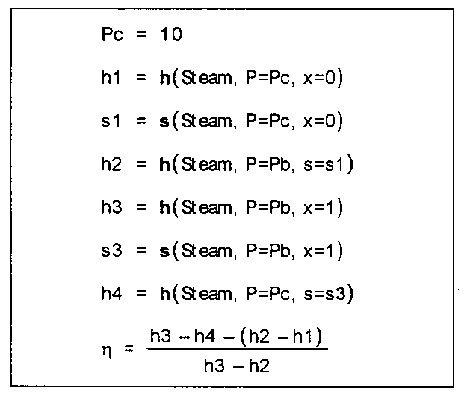
To illustrate this program, consider an ideal Rankine steam cycle with a condenser pressure of 10 kPa. Lets say that we want to study the effect of boiler pressure on the cycle efficiency. Then in the EES program the equations are first typed in as follows,
| {Ideal Rankine steam cycle operating between
pressures Pb and Pc}
Pc=10 {kPa} h1=Enthalpy (Steam,p=Pc, x=0.0) s1=Entropy (Steam,p=Pc, x=0.0) h2=Enthalpy (Steam,p=Pb, s=s1) h3=Enthalpy (Steam,p=Pb, x=1.0) s3=Entropy (Steam,p=Pb, x=1.0) h4=Enthalpy (Steam,p=Pc, s=s3) eta=((h3-h4)-(h2-h1))/(h3-h2) |
These equations in Formatted form (an option supplied within EES) look as follows,

Next a Parametric Table is set up to evaluate the effect of boiler pressure on the efficiency,
| Pb (kPa) | eta |
| 1000 | |
| 2000 | |
| 3000 | |
| 4000 | |
| 5000 | |
| 6000 | |
| 7000 | |
| 8000 | |
| 9000 | |
| 10000 |
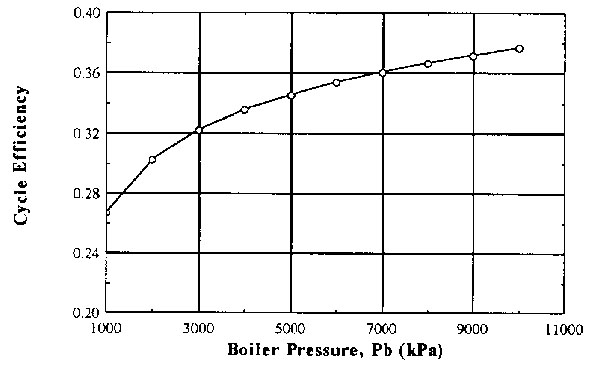
This Parametric Table is solved next which yields the following results,
| Pb (kPa) | eta |
| 1000 | 0.267 |
| 2000 | 0.303 |
| 3000 | 0.322 |
| 4000 | 0.336 |
| 5000 | 0.346 |
| 6000 | 0.354 |
| 7000 | 0.361 |
| 8000 | 0.367 |
| 9000 | 0.372 |
| 10000 | 0.377 |
As expected the cyclic thermal efficiency increases with increasing boiler pressure. If a plot is desirable then it is possible to prepare a plot within EES as follows,
This illustrates the advantages of this particular computer aided instruction. As seen, all of the above information was generated within EES and transported to the word processor for the purpose of documentation.
The program can also be utilized for trial and error analysis via minima-maxima calculations. Lets say that in the above example we want to know what the boiler pressure will have to be to yield an efficiency of 40%? Then the problem may be set up as,
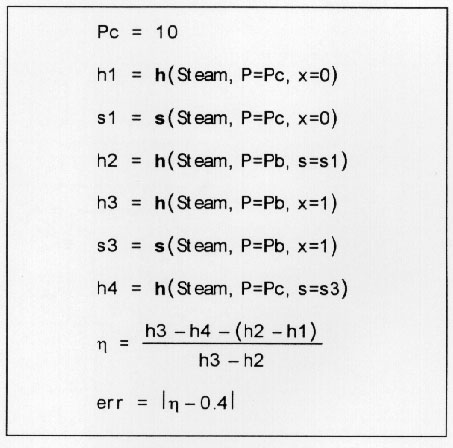
Next the error is minimized while varying boiler pressure within the user specified bounds. This yields an answer of 14,853.5 kPa for the boiler pressure which can be easily verified.
CAUTION WITH COMPUTERS IN ENGINEERING EDUCATION
While the computer aided education is desirable, if not essential, there are some concerns which we would like to address. Computer is only a tool and hence is not a substitute for critical thinking. While computers are great for exploration and for performing "what-if" types of analyses, they can hinder the process of creative query and common-sense compatibility. Armed with computers the students seem eager to get answers fast and to trust those results without question or hesitation. Occasionally when they are not certain about the results, they have a difficult time in rectifying any potential conceptual or mathematical flaws. Some examples and proposed remedies are discussed in this section with a few concluding remarks about an article which the author read, "Beware of the cosmetic engineer!" [2].
Continuing on with the above example, lets say that we want to determine the required boiler pressure for obtaining cyclic efficiencies of 41%, 43% and 45%. Most students will present the following results based on the previous program output:
| eta | Pb (kPa) |
| 0.40 | 14853.5 |
| 0.41 | 18614.0 |
| 0.43 | 19970.5 |
| 0.45 | 19970.5 |
At this point if I would ask them, "Why are you getting multiple efficiencies at the same pressure of 19970.5 kPa?" then through discussions the following facts emerge: First, the efficiency is actually 'capped' off at 41.3% but they failed to notice that the err was never zero for efficiencies above 41.3%. Hence, what they obtained was an efficiency of 41.3% regardless of what they were seeking. Secondly, the specified lower & upper bounds were 1 MPa and 20 MPa respectively. As a next step when they change the upper bound to 30 MPa, they obtain the following answers,
| eta | err | Pb (kPa) |
| 0.40 | 0.0 | 14853.5 |
| 0.41 | 0.0 | 18614.0 |
| 0.42 | 0.0 | 22998.4 |
| 0.43 | 0.005 | 26554.3 |
| 0.45 | 0.025 | 26554.3 |
At this point then I discuss the Critical Pressure for water being 22.1 MPa and it's implications. This is one example of why caution is needed before the computer aided results can be trusted.
One common pitfall I have observed with all the equation solvers is their inability to recognize existence of multiple roots. Now, if I ask the students about a particular equation and the roots, most of them will recognize the possibility of multiple roots and most will be able to identify those roots that are physically possible. However, once they start dealing with a set of equations they seem to overlook this possibility and worst yet when they are unable to figure out why their results do not make sense! It should be noted that their 'results' include only the quantities of their interest. But when one looks at values of all different parameters the absurdities become self evident! Examples include: negative mass flow rates, temperatures below 0°K, negative pressures or times or volumes etc., temperatures in excess of million degrees, violation either of the second law of thermodynamics or of the stability criteria along the way, so on and so forth.
Another particular difficulty I have noticed is when the students are given actual, experimental data to work with. As an example when I gave the following problem in my heat transfer course, I got very interesting and differing answers.
PROBLEM: Consider a flow of incompressible fluid through a 100 mm diameter circular tube. The table below gives the actual measured values of temperature and axial velocity at various locations. The fluid properties are: m = 0.25 N.s/m2, r = 1000 kg/m3 and k = 0.5 W/m°K.
| Radius (mm) | Temperature (°C) | Velocity (m/s) |
| 0.0 | 350.0 | 10.0 |
| 16.0 | 345.9 | 8.9 |
| 32.0 | 330.1 | 5.9 |
| 48.0 | 303.9 | 0.8 |
| 50.0 | 300.0 | 0.0 |
a) What is the temperature at the wall?
b) What is the mean velocity and mean temperature?
c) What is the Reynolds number? Is the flow laminar or turbulent?
d) What is the wall heat flux and it's direction?
e) What is the wall shear stress and it's direction with respect to the flow?
f) What is the heat transfer coefficient and the Nusselt number at this location?
For sake of discussion I will consider only the first two questions here. For part (a), while most students seemed to have understood the materials presented in the class and in the textbook, some still had difficulty in 'orienting' themselves in realizing that the wall is where the velocity is zero or where the radius is maximum. For the part (b), most had difficulty in numerically evaluating the following integrals and that's where a rather wide range of answers and approaches were observed:
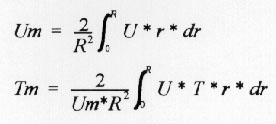
While some used the numerical integration option that is within EES, most others used the trapezoidal approach for integration. The difficulty arose when they tried to define U, T and r over a given range of dr.
CONCLUSIONS
Integration of computers in engineering education is indeed a welcome and timely change. We strongly endorse and recommend use of computer aided instruction. However, we also caution against seemingly blind acceptance of computer technology. Attention is brought to a particular article entitled, "Beware of the cosmetic engineer!" which describes the pitfalls when show is given priority over the substance. Critical thinking and analytical skills still should be fostered. Since computers make it easier to obtain answers, efforts should be made so that students are trained to ask good questions that are revealing and insight providing. A powerful combination of computational skills along with critical thinking abilities will produce students ready for the challenges of the 21st century.
REFERENCES
[1] Klein, S. A. and Alvarado, F. L., "Engineering Equation Solver," F-Chart Software, Middleton, Wisconsin (1995)
[2] M. Jones, "Beware of the cosmetic engineer", IEEE Spectrum, pp. 48, June 1993.